Insulation Treatments and Corrosion under Insulation: What Works
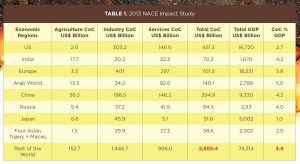
One of the most common concerns about industrial insulation systems is the risk of corrosion under insulation (CUI). Many papers and studies have been published regarding the financial impact of CUI on different sectors of the U.S. and global economies. The 2013 National Association of Corrosion Engineers (NACE) Impact Study estimated the cost of CUI in the U.S. industrial market segment alone at more than $300 billion (Table 1). On a global scale, across all economic sectors, the cost of CUI was estimated at more than $2.5 trillion in 2013, not including the current economic impacts. Table 1 shows the cost of corrosion (CoC) across industries and geographies. Note that NACE is now known as the Association for Materials Protection and Performance.
This article covers the factors that can cause CUI and the elements of an effective corrosion prevention strategy. It also looks at commonly found types of industrial insulations—water repellent and hydrophobic insulations—and considers their effectiveness. Finally, it discusses the properties that have been proven to reduce the risk of CUI. Many insulation manufacturers focus on trying to prevent water from getting into the insulation system, but this article assumes that water will get into the system due to damage or the lack of maintenance, and therefore focuses on products with a corrosion inhibitor.
Understanding the Causes of CUI
To minimize the risk of CUI, it is important to understand the corrosion process. CUI is an
electrochemical process. For CUI to start and continue, a combination of the following must be present:
- Oxygen,
- Corrosive chemicals or compounds—pH < 7,
- A temperature at the metal surface between 25°F and 350°F (or a cyclic system that passes through this range on some regular frequency), and
- An electrolyte (liquid water) to “close” the anode/cathode pathway.
The challenge of preventing or slowing CUI lies in identifying a way to disrupt this mix of conditions. Since our atmosphere naturally contains oxygen, it is not possible to remove this part of the equation. Corrosive chemicals or compounds most often enter the insulation system from the external environment in the form of rainwater, acid rain, coastal fog, fallout from cooling towers, exhaust from coal-burning power plants, or chemical emissions from processes within the plant itself. As with oxygen, it is usually not possible to prevent exposure of the insulation system to corrosive materials. Additionally, the temperature of piping or equipment is that which is required for each given process area, and there is typically a very narrow window under which the process runs properly. Therefore, it is not possible to keep the pipe or equipment from operating within, or cycling through, the temperature corrosion zone.
Industry experts agree that the primary cause of CUI is water ingress into or condensation within the insulation system that migrates to the exposed metal surface beneath. This situation can occur due to poorly installed insulation and jacketing, damage to the system that allows water to enter, or just unusual weather conditions like severe storms or flooding that expose the system to atypical conditions.
Most experts in industrial insulation systems acknowledge that water is likely to penetrate the insulation system and get to the metal surface beneath the insulation. Here are a few reasons why that happens:
- Insulation in ambient temperature and above processes is not sealed at the longitudinal and circumferential seams so as to allow it to move to accommodate thermal expansion and contraction of the process piping or equipment and the insulation material itself.
- For processes operating above the boiling point of water, the insulation system must be able to breathe so that any water that does reach the hot pipe or equipment surface and evaporate can escape the system. It is important to also note that “breatheable” systems will also allow water vapor to enter the system, which can then condense on the exposed metal surface during temperature cycling or downtime.
- A properly installed metal jacketing system has the overlapped seams oriented to provide a natural watershed and oppose the prevailing winds. But on many horizontal pipe runs or equipment, it is impossible to determine the pitch of the pipe to know in which direction to orient the overlap. As wind direction frequently changes, it is often not possible to fully eliminate the chance for wind-driven rain to get beneath the metal.
- Even if the jacketing is initially installed perfectly, over time many situations can create openings in the metal and allow water to enter, such as frequent expansion/contraction causing overlapped seams to move apart and open; damage from facility personnel walking on the insulated pipes; and impact from vehicles, equipment, or dropped tools.
- Many hot thermal insulation systems incorporate expansion/contraction joints to compensate for differences in coefficients of expansion between the metal pipe and the insulation. These joints are most often packed with insulation that is not hydrophobic and therefore represents a weak spot for water intrusion into the system. They can then become paths for water to migrate to the metal surface beneath the insulation after it enters the system.
For the reasons detailed above, water is able to get inside the outer metal jacketing and migrate to the pipe or equipment beneath the insulation without passing through it.
A Systematic Approach to Mitigating CUI
In an ideal world, CUI mitigation is a systematic approach that must include the following elements.
- When process conditions allow, apply an appropriate coating to the metal surface to act as the last line of defense.
- Select insulation materials that minimize the risk of CUI.
- Ensure all insulation systems (insulation and jacketing) are properly installed to minimize the opportunity for water to enter (the first line of defense).
- Most importantly, conduct regular inspections of all insulation systems and repair any CUI damage found as quickly as possible.
- If the inspection process requires removing sections of jacketing and insulation to inspect the underlying metal, these inspection sites must be repaired promptly so they do not then become a weak spot in the system and allow future water ingress.
Water Repellent/Hydrophobic Insulation Materials
A common approach being promoted today to keep water out of the insulation system and away from the underlying metal surface is the use of water repellent or hydrophobic insulation materials. It makes sense that if the insulation material helps to slow or prevent the migration of water from the outside environment to the metal surface beneath the insulation, this will prevent the commencement of CUI. Unfortunately, several important factors must be considered.
- The terms “water repellent” and “hydrophobic” do NOT mean the material has zero permeability. They simply mean the material will absorb less water and do so more slowly than those that are not water repellent or hydrophobic. Over time, with repeated contact, some water will still be absorbed into the insulation.
- Damage to the insulation and jacket system over time, combined with a lack of repair/maintenance work, creates multiple opportunities for water to migrate to the metal surface beneath the insulation. Water does not have to pass through the insulation material itself to reach the metal surface and trigger the corrosion process.
- Insulations are made water repellent or hydrophobic by the inclusion of organic compounds (silicones) in their chemistry. These additives all have an oxidation temperature somewhere between 400°F and 600°F. If the process operates at, or cycles to, temperatures above that range, these organic compounds will oxidize and decompose, rendering the portion of the insulation exposed to the higher temperatures ineffective at repelling water.
Inhibiting Corrosion at the Molecular Level
If we look back at the conditions needed for corrosion to be triggered, we must revisit how we can further break the corrosion cycle. The answer is to look at the chemistry of the insulation material being used and how it reacts with the water that will eventually work its way to the metal pipe or equipment surface.
Johns Manville Thermo-1200® calcium silicate and Sproule WR-1200® perlite both contain a proprietary corrosion-inhibiting formulation: XOX. The XOX Corrosion Inhibitor® is part of the chemistry of each product and is dormant as long as the insulation system is dry. However, when water enters the system and comes in contact with the insulation material, it activates the corrosion-inhibiting compounds and creates two levels of protection.
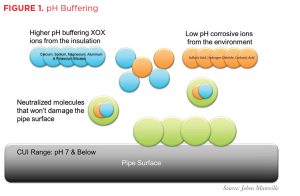
Remember that one of the conditions necessary for CUI to begin is the presence of corrosive (low-pH) ions that typically enter the insulation system from the external environment in rainwater. When the water carrying these corrosive ions comes in contact with insulation that contains corrosion-inhibiting compounds, it picks up slightly alkaline ions from the compounds. These buffering ions neutralize the acidic, corrosive ions and work to keep the insulation system, including the metal pipe or equipment surface, at a neutral pH, thereby minimizing the risk of corrosion starting (see Figure 1).
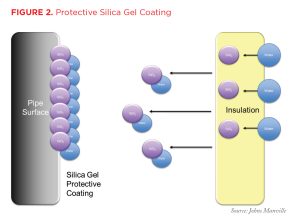
The second, and more powerful, level of protection is the formation of a protective passivation layer on the surface of the metal beneath the insulation. When water migrates through and/or around the insulation system and collects at the insulation/metal interface, it dissolves molecules of the silicate ions in the corrosion-inhibiting package. These ions then collect on the metal surface, forming a protective “silica gel” coating, and react with iron compounds on the metal surface to form inorganic iron silicate that helps stabilize and toughen the coating (see Figure 2).
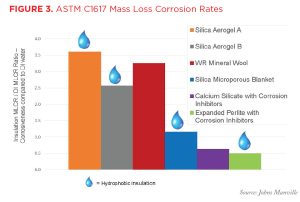
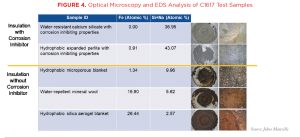
The effectiveness of these corrosion-inhibiting packages has been verified by testing conducted at independent, third-party laboratories according to ASTM C1617, Standard Practice for Quantitative Accelerated Laboratory Evaluation of Extraction Solutions Containing Ions Leached from Thermal Insulation on Aqueous Corrosion of Metals. This test method details a procedure for calculating a mass loss corrosion rate that is solely dependent on the chemistry of the tested insulation material as the catalyst for the corrosion. The data presented in Figure 3 clearly show that insulation materials with corrosion inhibitors demonstrated a strong ability to prevent CUI, as their mass loss corrosion rate ratios (compared to deionized water [DI]) were less than one. It also shows that insulation with hydrophobic properties can still have a high mass loss corrosion rate. Note that in Figure 3, the lower the mass loss corrosion ratio, the better the material performs in reducing the risk of CUI.
Additional optical microscopy and electron diffraction spectroscopy (EDS) analysis of the test samples from the ASTM C1617 testing further validated the actual performance of the corrosion inhibiting package and verified that corrosion occurred with insulation materials not containing any corrosion inhibitors. As shown in Figure 4, analysis of the calcium silicate and perlite insulation samples (which contained corrosion inhibitors) identified a surface layer of silicate (Si) and sodium (Na). This is the protective “silica gel” layer formed when the corrosion-inhibiting compounds were activated. More critically, analysis of the samples for the silica aerogel and mineral wool insulations, products with no corrosion inhibitors, identified a very high concentration of atomic iron (Fe), or rust.
Conclusion
It is important to understand the mechanisms of CUI so that truly effective mitigation methods can be selected and employed. It also is critical to approach any CUI prevention strategy as a complete system solution and not look for a single, silver bullet cure.
With the understanding that water will eventually penetrate many insulation systems and come in contact with exposed metal surfaces beneath the insulation, relying solely on the insulation material to keep water away from the metal surface is not an effective approach. Additionally, since that water often contains corrosive compounds, using insulation materials that contain corrosion-inhibiting chemistry that will both neutralize the acidic contaminants and create a protective silica passivation layer on the metal pipe or equipment surface is an effective solution.